The world of chemistry is based on interaction between countless molecules. While some are complex, basic things often turn about simple, abundant connections. These include hcooch ch2 h2o — formic acid, methylene group (CH) and water. Separately, they are corners of organic and inorganic chemistry. Together, the chemical interaction of hcooch ch2 h2o also inspires reactions in essential industrial processes, biological functions, and even interstellar environments. Understanding their characteristics and reactions opens a window in everything from green chemistry to material science.
This post will detect the unique properties of hcooch ch2 h2o and their respective chemical institutions. We will examine the molecular structure of hydrogen-containing compounds, including hcooch ch2 h2o, and explore their roles in everything from polymer synthesis to renewable energy. When we see their conversation, we may appreciate their collective meaning in both industrial surroundings and natural world.
The Fundamental Building Blocks
To understand how these components interact, we should first look at them personally. Each has different properties that define its role in chemical reactions.
HCOOH: More Than Just an Ant Sting
It is systematically referred to as formic acid, or metanic acid, the simplest carboxylic acid. The chemical structure of hcooch ch2 h2o reveals a single hydrogen atom connected to a carboxyl group (-COOH). This structure of hcooch ch2 h2o, with its functional groups, gives formic acid its acidic properties. While many people know that formic acid is found in the poison of ants and bees, its significance extends far beyond nature.
In industrial applications, formic acid is a versatile tool. It is used as a preservative and antibacterial agent in livestock feed. In the textile industry, it acts as a dye and finishing agent. It also plays a role in leather brown and in rubber production. The format tester, when the formic acid reacts with an alcohol, is an important component of various chemical synthesis.
CH₂: The Versatile Methylene Group
The CH₂ component of hcooch ch2 h2o, known as a methyling group or methylene bridge, is not a stable standalone molecule under most conditions, but serves as a basic structural unit in organic chemistry. It consists of carbon atoms associated with two hydrogen atoms. This group acts as a lining, which combines functional groups to create the spine in countless organic compounds. You can find anything from simple hydrocarbons to complex polymers.
The importance of the methylene group is most obvious in polymarket. Polyming reactions, such as used to make biodegradable plastic, are often dependent on connecting monomers with mythylene bridges. Its presence is important in the synthesis of the materials we used every day. In addition, Choy intermediate recycling is a growing area of interest for lasting chemical production.
You can also read about australian pairs
H₂O: The Universal Solvent
Water is a simple molecule with deep effects. A water molecule is made of an oxygen atom bound to two hydrogen atoms. “How many oxygen atoms are in the water molecule?”The answer is one, but both atoms (in this case oxygen and hydrogen) have spatial events that we have chemical properties we have.The scheme creates polarity and allows for strong hydrogen bonding.
The ionic character of water allows it to function well as a solvent so that it can dissolve more chemicals than any other liquid. Water solvent properties are important in many chemical processes in research laboratories and industrial buildings. Water is also a favorite resolution in green chemistry because it is not -toxic and environmentally friendly. It is the medium for many reactions, including sour hydrolysis and basic hydrolysis, and is fundamental to all known biological systems.
Chemical Interplay and Reaction Mechanisms
The actual ability of these compounds is unlocked when they interact. The combination of hcooch ch2 h2o is central to many important reactions, especially in organic synthesis.
Ester Hydrolysis and Formation
One of the most classic reactions involving these components is the inverse of esters hydrolysis, esteem. An ester, such as methyl format, can be divided into an alcohol in the presence of a carboxylic acid and water. This reaction can be catalyzed by acidic conditions, often by using catalyst acids such as sulfuric acid or basic conditions. The reaction system involves a nucleophilic replacement in the carbon carbon to esters.
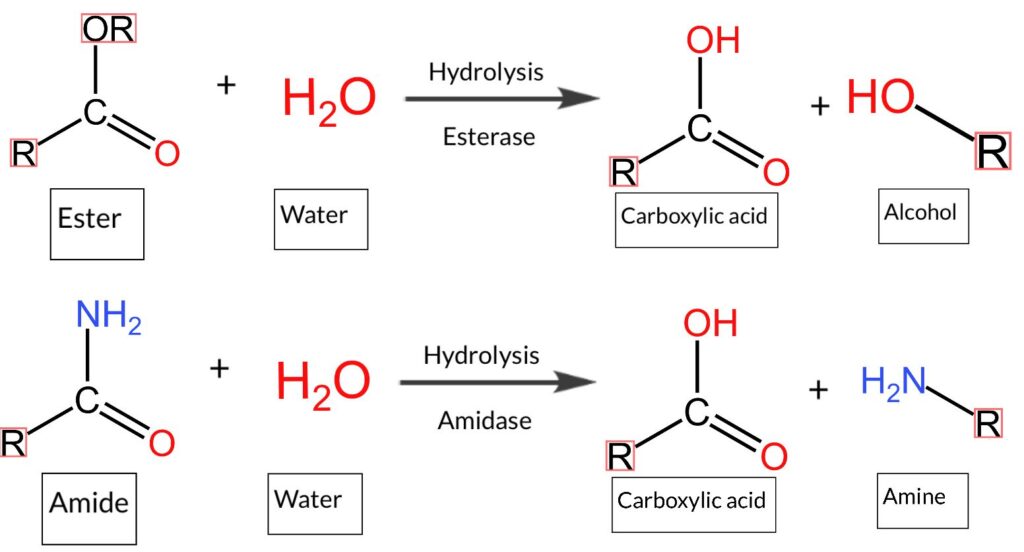
For example, hydrolysis of an ester that has a methylene group will produce formic acid (if there is a format tester) and an alcohol. The response rate is affected by the presence of temperature, pH and catalyst. This process is important in biodiesel production, where estropes in triglycerides are damaged.
Polymer Synthesis and Industrial Applications
Chemical interaction between these substances is also in the heart of polymer formation. Combination reactions, where two molecules together are associated with damage to a small molecule such as water, are common. For example, formic acid may react with a compound containing a hydroximethyl group (–Ch₂OH), forming an ester binding and releasing water.
In industrial environments, these reactions are expanded for chemical construction. Understanding the status of response dividends and adaptation are the most important functions for chemical engineering science. These procedures are used to create materials for everything from consumables to advanced technologies. The principles of aquatic chemistry are used to develop more durable and effective industrial synthesis methods.
You can also read about mike wolfe passion project
Environmental and Advanced Applications
Push for green technology and renewable resources has focused a new focus on compounds such as formic acid, water and methylene -containing molecules. Their roles in environmental science and energy are expanded rapidly.
Green Chemistry and Sustainability
Maursic acid is considered a valuable vector for hydrogen storage, which is an important challenge in developing fuel cell technologies. Fuel cells generate energy through redox reactions, and formic acid can be solved to release hydrogen gas and carbon dioxide. This creates a promising component in the step against renewable energy.

The role of water in green chemistry cannot be eliminated. As a solvent, it eliminates the requirement for volatile organic compounds that are harmful to the environment. In addition, procedures such as hydration of alkanes use to produce alcohol often water as retirement. It is necessary to make feedstox for biodegradable plastic and other durable materials.
From Prebiotic Earth to Modern Materials
The importance of these simple molecules can also increase to the origin of life. Scientists have shown formic acid and molecules with carbon oxygen compounds in intersteller clouds and in icy moon. Gas-stage reactions in these extreme environments, sometimes involving cryogenic catches, prebiotics can contribute to the formation of more complex organic molecules on earth.
Back to our planet, material science continues to find new uses for these basic components. From organometallic chemistry to nanotechnology, hcooch ch2 h2o, the principles that control interactions are used to design new compounds with specific properties.
Safety and Handling
Handling responsible with great use is required. Midic acid, although useful, is corrosive and requires careful handling. Guidelines for safety and handling should be followed, including appropriate safety equipment such as gloves and glass use, especially in industrial production.Understanding safety ideas for all chemicals involved in a reaction is a fundamental part of laboratory research and production.
Conclusion
The story of hcooch ch2 h2o is an ideal example of how simple chemical building blocks create a world of complexity and possibility. From the molecular structure to hydrogen bonds in water to the role of a polymer recycling methyling group, these components are everywhere. They run industrial processes, provide solutions for a green future and are integrated parts for biological systems. Continuing to detect their chemical interplays, researchers in chemical engineer Nano Science technology and related fields will undoubtedly unlock even more applications that benefit both industry and the environment.